nitrogen valence electrons
The valency of a nitrogen atom is 3 5 and the valence electrons of a nitrogen atom are five. Apart from that one more thing is unique about the.
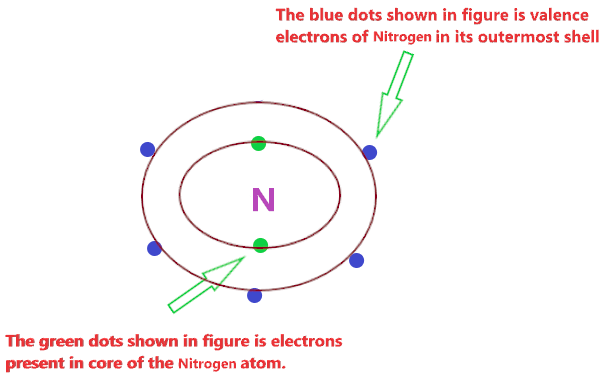 |
| Is No2 Covalent Or Ionic Or Both Types Of Bond In No2 |
The second method to find the valence electrons of the.

. Nitrogen is the lightest element in this group having an atomic number of 7. In methane carbon has a valence of 4. From this electronic configuration we come to know that the number of electrons present in. Nitrogen has five valence electrons.
There are five valence electrons in nitrogen atom and they always participate in covalent bond formation. Nitrogen is in Group 15 also called 5A and therefore Nitrogen has five valence electrons note that this method doesnt work for the Transition Metals. And the total number of electrons present in this energy level is 2. A valence electron is the total number of electrons found in a valenceshell.
Nitrogen Electron Configuration The last shell of nitrogen has three unpaired electrons so the valency of nitrogen is 3. The combining capacity or affinity of an atom of a given element is determined by the number of hydrogen atoms that it combines with. What is the number of valence electrons in nitrogen. The type of bonding that atoms of elements are involved in depends.
The last electron of nitrogen enters the p-orbital. And the last shell has a total of five electrons. 119 rows Valence electrons in Nitrogen N 5. We know the electron configuration of the Nitrogen atom is 1s 2 2s 2 2p 3 and valence electrons are those electrons found in the outer shell of an atom.
You can see in the electron configuration of nitrogen 1s2 2s2 2p3 that the highest energy level is 2. In the case of nitrogen the highest value of n is 2. The last shell of nitrogen has three unpaired electrons so the valency of nitrogen is 3. There are 2 ways to find the number of valence.
Nitrogen has five valence electrons. You can also look at the electron. Therefore the valence electrons of nitrogen are five. Therefore it can accept 3 electrons to complete its octet.
Valence electrons in Neon Ne 8. Usually nitrogen forms three covalent bond by employing its unpaired electrons. The electronic configuration of nitrogen is 1s22s22p3. The atomic number of nitrogen element is 7 and its electronic configuration is.
So in n 2 you. The valence electrons of each main group element can be determined by the column in which it is located. Valence electrons in Fluorine F 7. The electron configuration for nitrogen shows that the last nitrogen shell has five electrons.
Valency of Nitrogen N is 3 as it has 5 electrons in its valence shell. In a nitrogen molecule a triple covalent bondis represented by three lines between two atoms of. This electron configuration of nitrogen. To find the valance electron you have to consider the highest value of n or the principal quantum number.
Ie all group 1 elements have 1 valence electron all group 2. Nitrogen has five valence electrons three of which are unpaired and the other two form a lone pair. Nitrogen atoms are the 2nd period of the periodic table and an element of the 15-group. There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table.
Valence electrons in Oxygen O 6.
 |
| How Is Oxygen Atom Bonded To Carbon Atom In Co3 2 |
 |
| N2 Lewis Structure In 6 Steps With Images |
 |
| How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube |
 |
| Premium Vector Diagram Representation Of The Element Nitrogen Illustration |
 |
| Solved How Many Valence Electrons Does A Nitrogen Atom Have Chegg Com |
Posting Komentar untuk "nitrogen valence electrons"